Molecules:
- The smallest particle of an element or a compound that is capable of an independent existence and shows all the properties of that substance is said to be Molecules.
- Also we say, it is a group of two or more atoms that are chemically bonded together or tightly held together by attractive forces.
- Either atoms of the same element or of different elements can join together to form molecules.
- The molecules of an element constitutes same type of atoms.
Atomicity:
- The number of atoms constituting a molecule is said to be its atomicity.
- Atoms and molecules notes for class 9 deals with atomicity in detail.
Molecules are classified into 4 categories based on the number of atoms present in it:
- Monoatomic: Molecules which contains only one atom is said to be monoatomic. Example: He, Ne, Ar etc.
- Diatomic: Molecules which contain two atoms are said to be diatomic. Example: O2, H2, Br2 etc.
- Triatomic: Molecules which contain three atoms are said to be triatomic. Example: O3, NO2, CO2 etc.
- Tetra atomic:Molecules which contain four atoms are said to be tetra atomic. Example: P4, SO3 etc.
Molecules are further divided into two types:
- Homo-atomic Molecule: If the molecule is constituted with only single type of atoms then it is said to be Homo-atomic molecules. Learn and revise Atoms and Molecules Notes to score better.
| Name |
Atomicity |
| Argon |
Mono-atomic |
| Helium |
Mono-atomic |
| Oxygen |
Di-atomic |
| Hydrogen |
Di-atomic |
| Nitrogen |
Di-atomic |
| Chlorine |
Di-atomic |
| Phosphorous |
Tetra-atomic |
| Sulphur |
Poly-atomic |
- Hetero-atomic Molecule: When different type of atoms combine to form a molecule then these molecules are said to be hetero-atomic molecules. We have provided you with examples in atoms and molecules notes.
| Compound |
Combining Elements |
Atomicity |
| Water |
Hydrogen, Oxygen |
Tri-atomic |
| Ammonia |
Nitrogen, Hydrogen |
Poly-atomic |
| Carbon dioxide |
Carbon, Oxygen |
Tri-atomic |
Ions:
- Charged Particles are called ions.
- They can have positive or negative charge on it.
- Negatively charged ion are known as anion. Example: Cl-, O2- etc.
- Positively charged ion are called cation. Example: Na+, K+ etc.
Valency:
- The combining capacity of an element is known as its valency.
- The main use of valancy is to find out how atom of an element will combine with the atom of another element to form a chemical compound.
- To gain stability, atoms either gains or loose or shares its electron. Read atoms and molecules notes below for more.
- If there is 1, 2, 3 electrons are their in valance shell then its valency is 1, 2, 3 respectively as it can lose its electron.
- If there is 5, 6, 7 electrons in their valance shell then its valency will be 3, 2, 1 respectively as they will gain the electrons to become stable.
- If an atom has 4 electrons in the valance shell than it will share its electron and hence its valency will be 4.
- The valency of an element is 0 if an atom has 8 electrons in the outermost shell.
Remember: “All polyatomic ions with names starting with ‘S’ has valency 2 and CO32- has 2 and PO42- has valency of 2 otherwise each element has valency of 1.”
Revise atoms and molecules notes regularly.
Chemical Formulae:
Rules for writing Chemical formulae:
- First of all, the charges or valency of cation and anion must be balanced.
- If the compound contains both metals as well as non-metals then, write their symbols first.
(As, H + Cl → HCl)
- When ions are polyatomic then we indicate its number along with charge by separating bracket.
[As, (SO4)2-, Mg(OH)2]
- Learn and revise atoms and molecules notes wisely.
Chemical Formula of some simple compounds:
- While writing the chemical formulae for compounds, we crossover the valencies of the combining elements. Read carefully atoms and molecules notes.
- Formula of hydrogen chloride or Hydrochloric acid:
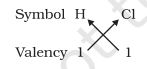
- Formula of hydrogen sulphide:
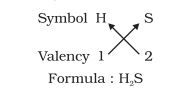
- Formula for aluminium oxide
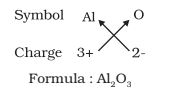
- Formula of calcium hydroxide:
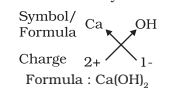
Molecular Mass:
- The sum of the atomic masses of all the atoms in a molecule of the substanceis said to be molecular mass.
- It is also expressed in atomic mass unit (amu).
- The other subsection of atoms and molecules notes is here.
Formula Unit Mass:
- The sum of the atomic masses of all atoms in a formula unit of a compound is said to be formula unit mass of a substance.
- It is similar as the molecular mass but the only difference is that in this types of compounds constituent particles are ions rather than atoms.
- Example: In NaCl, 1 x 23 + 1 x 35.5 = 58.5 u
- Further we will read in higher section of atoms and molecules notes.
Mole Concept:
- We describe quantity of substance with the help of mole.
- Mole: The amount of substance which contains 6.023 x 1023 units is said to be 1 mole.
- The mole is the amount of substance that contains the same number of particles (atoms/ ions/ molecules/ formula units etc.) as there are atoms in exactly 12 g of carbon-12.
- 1 mole = 6.022 x 1023 units = 1 NA (NA is Avogadro number).
- Example: 1 mole of Oxygen = 6.023 x 1023 atoms of oxygen.
- Atoms and molecules notes contains a part from mole concept from NCERT.
Q/A on atoms and molecules notes:
To understand it better here are some examples from NCERT class 9 chapter 3 notes. Let’s revise them as well:
Calculate the relative molecular mass of water (H2O).
Solution:
As we Know that,
Atomic mass of hydrogen (H) = 1u, oxygen(O) = 16 u
So the molecular mass of water, which contains two atoms of hydrogen and one atom of oxygen is = 2 × 1+ 1×16 = 18 u
Calculate the formula unit mass of CaCl2.
Solution:
Formula unit mass of CaCl2 =
Atomic mass of Ca + (2 × atomic mass of Cl)
= 40 + 2 × 35.5 = 40 + 71 = 111 u
Calculate the mass of 0.5 mole of O2 gas.]
Solution:
Mass = molar mass × number of moles
⇒ 32 x ½ = 16 g
Calculate the mass of 6.022 × 1023 number of O2 molecules.
Solution:
As we know that
n = \(\frac{ given\:umber\: of\: particles}{Avogadro\: number}\) = \(\frac{N}{N_{A}}\)
n = \(\frac{6.022 × {10}^{23}}{6.022 × {10}^{23}}\)=1
Atoms and molecule notes for class 9 thus provides you with the actual curriculum what CBSE and other boards follow. Read, learn and revise it regularly.