A.C Charles; in 1787; studied the variation in volume of gas with temperature; pressure remaining constant. According to Charle’s law.
at constant pressure; the change in voulme (ΔV) per unit volume at 0°C (V0) per degree change in temmperature is a constant. Let V0 and V denote the volume of gas at 0°C and θ°C at constant pressure, then

γp is known as coefficient of volume expansion of gas at constant pressure. Experiments show

γp = 1/273.15. Eqn. (1) can be written as
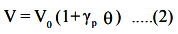
This law is also referred as law of volume. Eqn (2) is rewritten as
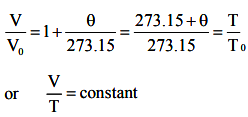
T0 and T denote absolute temperature of gas at 0°C and θ°C respectively