Binding energy of nuclides is given by the equation
B( AZX) = [Nmn + ZM(1H) – M ( AZX) ]c2
On dividing binding energy by the mass number, we obtain the binding energy per nucleon,

= [10 × 1.008665 + 10 × 1.007825 – 19.992440 ] × 931.5
= 160.6 MeV
Hence binding energy per nucleon = B(2010Ne)/20
= 8.03 MeV/nucleon

= [30 × 1.008665 + 26 × 1.007825 – 55.93492] × 931.5 = 492 MeV
Hence binding energy per nucleon =B(5626Fe)/56
= 8.79 MeV/nucleon

Hence binding energy per nucleon
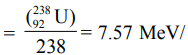
nucleon. All three nuclides have a binding energy per nucleon nearly 8 MeV, with 56Fe having the largest binding energy per nucleon.