First we find out the molecular formula of the compound
% of O2 = 100 - (% of C + % of H) = 100 - (69.77 + 11.63)
= 100 - (81.40)= 19.6%
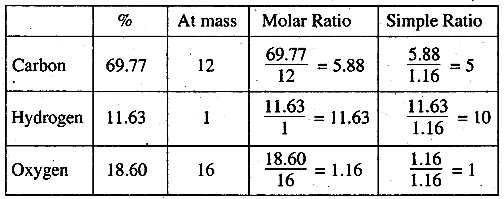
Empirical formula of the compound = C5H10O
Empirical formula mass = 5 × 12 + 10 × 1 + 16 × 1 = 86
but given that molecular mass = 86
n = \(\frac{molar\;mass}{Emperical \;formula} = \frac{86}{86} = 1\)
∴ molecular formula of the compound (C5H10O),
= C5H10O
Now we will find the structure of the compound
It is given that
1. It does not reduce Tollens’ reagent but forms addition compound with sodium hydrogen sulphide. As we know that only methyl ketone and aldehydes react with sodium hydrogen sulphide, the compound is methyl ketone. Also given that it given iodoform test. Thus the given compound can be represented as
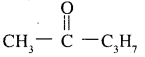
2. On vigorous oxidation it gives ethanoic acid and propanoic acid. This shows that the propyl group attached to >C = O group in n-propyl and not isopropyl. So the compound is
